Water boils at 212 degrees Fahrenheit or 100 degrees Celsius but begins to evaporate at 32 degrees Fahrenheit or 0 degrees Celsius only slowly. Solvents that boil over 50C are preferred.

Evaporation Chemistry For Non Majors
This technique can be used to separate a mixture of solids one of which can undergo sublimation.
. Evaporation is the movement of a substance from its liquid to its vapor phase. In simple distillation a mixture is heated and the most volatile component vaporizes at the lowest temperature. Distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated.
Less volatile substances. While the word temperature is commonly used to describe how hot or cold something is in terms of the kinetic molecular theory temperature is _____. If liquid evaporates what does it become.
The vapor passes through a cooled tube a condenser where it condenses back into its liquid state. While the external energy of an object considers the total potential and kinetic energy that is easily observed the _____ energy of. The vapours are then cooled separately to get the sublimed solid back a process called deposition.
Liquids with low boiling point evaporate quickly due to high vapour pressure. When water is heated it evaporates. Evaportaion happens as a liquid transforms to a gaseous state.
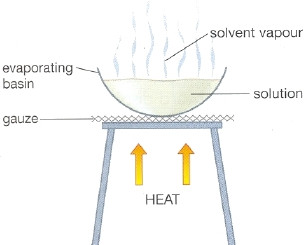
The same goes for acetone and water. An example of this type of mixture is sugar in water. Once again safety edict that this flask never is heated when covered or capped with stopper.
Heat from the sun or solar energy powers the evaporation process. The answer is molecules do both but under different circumstances notably the heating you have introduced. As the temperature increases the rate of evaporation also increases.
A mixture in which a substance has fully dissolved in water is called a solution and the substance is said to be soluble. Evaporation is one of the phases of the water cycle. The word solution is used to describe a liquid or solid phase containing more than one substance when for convenience one of the substances which is called the solvent and may itself.
Volatile liquid simply means that it evaporates easily. Good examples of these types of liquids include water H 2 O and mercury Hg. The rate of evaporation depends on the room temperature and a property of liquids called vapor pressure.
The word vapor is used to describe gases that are usually liquids at room temperature. The water vapor mixes with the air. List at least five terms from the introduction that you can use as key words in your search.
The opposite of evaporation is condensation. Movement directly from solid to vapor phase is called sublimation It is commonly used to concentrate solutions of nonvolatile solutes and volatile solvents. If liquid evaporates it will become steam.
These are capable of readily changing from a solid or liquid form. The noun volatile can also refer to elements or molecules that have low boiling points that easily evaporate such as water carbon dioxide nitrogen and hydrogen. When particles in the liquid phase are heated they gain kinetic energy and move faster and further apart.
It can be easily visualized when rain puddles disappear on a hot day or when wet clothes dry in the sun. Such liquids may be explosive others not. A liquid with a high vapor pressure can evaporate easily and it is called volatile.
Evaporation is the process when molecules from liquid pass to the atmosphere as gas without reaching the boiling point. 38K views Steve Ruis. It soaks up moisture from.
Evaporation is a very important part of the water cycle. Eventually they have enough energy to escape the forces of attraction holding them together in the liquid phase and they move very fast and far from each other and exist in the. This is an effect of evaporation or the change of matter from its liquid state to its vapour state.
Evaporation happens on a global scale. In these examples the liquid water is not actually vanishingit is evaporating into a gas called water vapor. Many liquids can evaporate but the question is what liquid can evaporate.
Evaporation happens when a liquid substance becomes a gas. The condition where molecules escape principally from the surface is an equilibrium condition where the temperature of the vapour and liquid are the same. Evaporation can generally be defined as a process by which a liquid or solid is transformed into vapour.
Evaporation is the process by which water changes its state from liquid to gas or vapor. Adding heat allows the liquid temperature to exceed that of the vapour to the extent that the liquid. It is also known as a boiling flask.
Evaporation happens when a liquid turns into a gas. When the molecules in a. Identify three websites you will use to start your research.
Solvents should not be volatile. If bubbles are formed we are talking instead about boiling. The water evaporates into water vapor the gas phase of water.
Florence flask contained a round bottom with a long neck. The rate of evaporation of a liquid may be increased by. They should not have low boiling points or evaporate easily.
Identifying Sources 5 points 1. When you spray perfume on your body your body feels slightly cooler. In evaporation a portion of the solvent is vaporized or boiled away leaving a thick liquid or solid.
Evaporation is a change of phase from liquid to gas explained as follows. For example water left in a bowl will slowly disappear. It is used for holding liquids and can easily be heated and swirled.
Open Yenka file Model 1 and add the solid to the beaker of water in each experiment. The molecules move and vibrate so quickly that they escape into the atmosphere as molecules of water vapor. Here the sugar dissolves fully to give a.
They get the vapor title when they are in a gaseous phase. In chemistry volatility is a material quality which describes how readily a substance vaporizesAt a given temperature and pressure a substance with high volatility is more likely to exist as a vapour while a substance with low volatility is more likely to be a liquid or solidVolatility can also describe the tendency of a vapor to condense into a liquid or solid. It can also be capped by glass or rubber stoppers easily.
Agitation is necessary for evaporation. Vapor and gas mean the same thing. If you use other websites to complete the research questions in Part 2 add them to this list.
You will probably hear the term water vapor which means water in a gas state. Evaporation is when a liquid becomes a gas without forming bubbles inside the liquid volume. 3 Sublimation is used in the separation of substances like ammonium.
Directly to vapour without passing through the liquid phase.
Why Do Liquids Evaporate When They Aren T At Their Boiling Temperature Quora
0 Comments